ARTISS

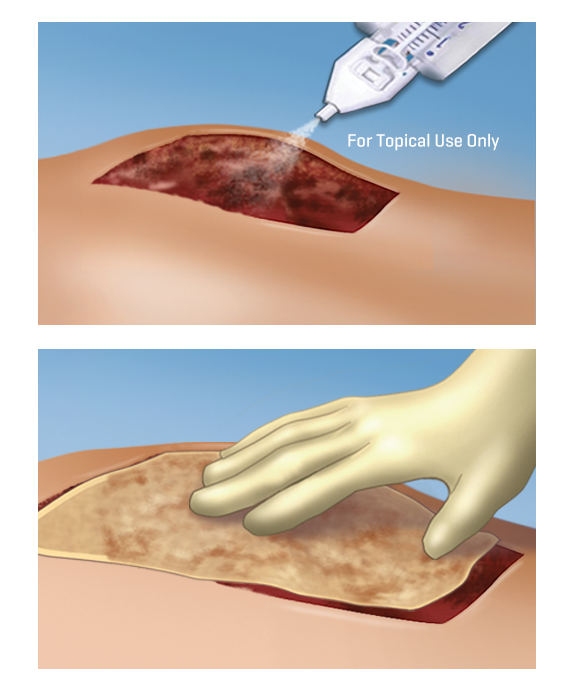
Mechanism of Action
ARTISS is a two component fibrin sealant, consisting of human thrombin and human fibrinogen and an antifibrinolytic inhibitor to delay clot degradation.2
Upon mixing, soluble fibrinogen is transformed into a fibrin matrix that adheres to the wound surface and to the skin graft to be affixed.2
Initial polymerization of ARTISS will take up to 60 seconds due to optimized thrombin concentration; allowing time to manipulate and position the graft prior to polymerization.2
The adhesive properties of ARTISS provide full surface adherence of the graft to the wound bed, closing the space that exists when
grafts are attached using point fixation techniques such as staples.1
ARTISS may reduce or eliminate the need for staple application or removal.1
This abbreviated summary of product characteristics (SmPC) is intended for international use. Please note that it may differ from the licensed SmPC in the country where you are practicing.
Therefore, please always consult your country-specific SmPC or package leaflet.
ARTISS, Solutions for Sealant, deep frozen
COMPOSITION
ARTISS consists of: • Human Fibrinogen (as clottable protein) 91 mg/ml • Synthetic Aprotinin 3000 KIU/ml • Human Thrombin 4 IU/ml • Calcium Chloride 40 μmol/ml
INDICATIONS
ARTISS is indicated as tissue glue to adhere/seal subcutaneous tissue in plastic, reconstructive and burn surgery, as replacements or adjuncts to sutures or staples. In addition, ARTISS is indicated as adjunct to hemostasis on subcutaneous tissue surfaces.
CONTRAINDICATIONS
ARTISS is not indicated to replace skin sutures intended to close surgical wound. ARTISS alone is not indicated for the treatment of massive and brisk arterial or venous bleeding. ARTISS must never be applied intravascularly. ARTISS is contraindicated in the case of hypersensitivity to the active substances or to any of the excipients.
SPECIAL WARNINGS AND SPECIAL PRECAUTIONS FOR USE
For epilesional use only. Do not apply intravascularly. Life threatening thromboembolic complications may occur if the preparation is applied intravascularly. Soft tissue injection of ARTISS carries the risk of local tissue damage.
Caution must be used when applying fibrin sealant using pressurized air or gas.
• Any application of pressurized air or gas is associated with a potential risk of air or gas embolism, tissue rupture, or gas entrapment with compression, which may be life-threatening or fatal
• Apply ARTISS as a thin layer. Excessive clot thickness may negatively interfere with the product´s efficacy and the wound healing process
• Life-threatening/fatal air or gas embolism has occurred with the use of spray devices employing a pressure regulator to administer fibrin sealants. This event appears to be related to the use of the spray device at higher than recommended pressures and/or in close proximity to the tissue surface. The risk appears to be higher when fibrin sealants are sprayed with air, as compared to CO2and therefore cannot be excluded with ARTISS when sprayed in open wound surgery
• When applying ARTISS using a spray device, be sure to use a pressure within the pressure range recommended by the spray device manufacturer.
• ARTISS spray application should only be used if it is possible to accurately judge the spray distance as recommended by the manufacturer. Do not spray closer than the recommended distances.
• When spraying ARTISS, changes in blood pressure, pulse, oxygen saturation and end tidal CO2should be monitored because of the possibility of occurrence of air or gas embolism
• ARTISS must not be used with the Easy Spray / Spray Set system in enclosed body areas
• Only use application devices CE marked for the administration of ARTISS
ARTISS is not indicated for hemostasis and sealing in situations where a fast clotting of the sealant is required. Especially in cardiovascular procedures in which sealing of vascular anastomoses is intended ARTISS should not be used.
ARTISS is not indicated for use in neurosurgery and as a suture support for gastrointestinal anastomoses or vascular anastomoses as no data are available to support these indications.
Before administration of ARTISS care is to be taken that parts of the body outside the designated application area are sufficiently protected/covered to prevent tissue adhesion at undesired sites.
Oxycellulose-containing preparations may reduce the efficacy of ARTISS and should not be used as carrier materials.
As with any protein-containing product, allergic type hypersensitivity reactions are possible. Signs of hypersensitivity reactions may include hives, generalized urticaria, tightness of the chest, wheezing, hypotension, and anaphylaxis. If these symptoms occur, the administration must be discontinued immediately.
ARTISS contains aprotinin. Even in case of strict local application, there is a risk of anaphylactic reaction linked to the presence of aprotinin. The risk seems to be higher in cases where there was previous exposure, even if it was well tolerated. Therefore any use of aprotinin or aprotinin containing products should be recorded in the patients’ records.
As synthetic aprotinin is structurally identical to bovine aprotinin the use of ARTISS in patients with allergies to bovine proteins should be carefully evaluated.
In the event of anaphylactic/anaphylactoid or severe hypersensitivity reactions, administration is to be discontinued. If possible, remove any applied, polymerized product from the surgical site. Adequate medical treatment and provisions should be available for immediate use in the event of an anaphylactic reaction. State-of-the-art emergency measures are to be taken. In case of shock, standard medical treatment for shock should be implemented.
Standard measures to prevent infections resulting from the use of medicinal products prepared from human blood or plasma include selection of donors, screening of individual donations and plasma pools for specific markers of infection and the inclusion of effective manufacturing steps for the inactivation/removal of viruses. Despite this, when medicinal products prepared from human blood or plasma are administered, the possibility of transmitting infective agents cannot be totally excluded. This also applies to unknown or emerging viruses or other pathogens.
The measures taken are considered effective for enveloped viruses such as human immunodeficiency virus (HIV), hepatitis B virus (HBV), and hepatitis C virus (HCV), and for the non-enveloped hepatitis A virus (HAV).
The measures taken may be of limited value against non-enveloped viruses such as parvovirus B19. Parvovirus B19 infection may be serious for pregnant women (fetal infection) and for individuals with immunodeficiency or increased erythropoiesis (e.g., hemolytic anemia).
It is strongly recommended that every time that ARTISS is administered to the patient, the name and batch number of the product are recorded in order to maintain a link between the patient and the batch of the product.
UNDESIRABLE EFFECTS
Intravascular injection could lead to thromboembolic events and disseminated intravascular coagulation (DIC) and there is also a risk of anaphylactic reactions.
Hypersensitivity or allergic reactions (which may include angioedema, burning and stinging at the application site, bradycardia, bronchospasm, chills, dyspnoea, flushing, generalized urticaria, headache, hives, hypotension, lethargy, nausea, pruritus, restlessness, tachycardia, tightness of the chest, tingling, vomiting, wheezing) may occur in rare cases in patients treated with fibrin sealants/hemostatics.
In isolated cases, these reactions have progressed to severe anaphylaxis. Such reactions may especially be seen if the preparation is applied repeatedly, or administered to patients known to be hypersensitive to aprotinin or any other constituents of the product.
Even if a first treatment with ARTISS was well tolerated, a subsequent administration of ARTISS or systemic administration of aprotinin may result in severe anaphylactic reactions.
Antibodies against components of fibrin sealant may rarely occur.
For safety with respect to transmissible agents, see section 4.4 of the SmPC.
Life threatening/fatal air or gas embolism when using devices with pressurized air or gas occurred; this event appears to be related to an inappropriate use of the spray device (e.g. at higher than recommended pressures and in close proximity of the tissue surface).
Adverse reactions summarized below were reported from clinical studies of ARTISS and from post-marketing experience with Baxter Fibrin Sealants (marked with a p in the adverse event table). Known frequencies of these adverse reactions are based on a controlled clinical study in 138 patients where skin grafts were fixed to excised burn wounds using ARTISS. None of the events observed in the clinical study were classified as serious.
The ADRs and their frequencies are: Common (³1/100 to <1/10) pruritus, skin graft failure; Uncommon (³1/1000 to <1/100): dermal cysts; Not known (cannot be estimated from the available data): Air embolism due to an inappropriate use of the spray device. Class Reactions:Other adverse reactions associated with products of the fibrin sealant/hemostatic class include: Hypersensitivity reactions which could manifest as application site irritation, chest discomfort, chills, headache, lethargy, restlessness and vomiting. Further class reactions are: Anaphylactic reaction, bradycardia, tachycardia, hypotension, haematoma, dyspnoea, nausea, urticaria, flushing, impaired healing, oedema, pyrexia and seroma. Reporting of suspected adverse reactions: Reporting suspected adverse reactions after authorisation of the medicinal product is important. It allows continued monitoring of the benefit/risk balance of the medicinal product. Healthcare professionals are asked to report any suspected adverse reactions via the national reporting system.
METHOD OF ADMINISTRATION
ARTISS is intended for Hospital Use only. The use of ARTISS is restricted to experienced surgeons who have been trained in the use of ARTISSFor epilesional (topical) use. Do not inject. For subcutaneous use only. ARTISS is not recommended for laparoscopic surgery.In order to ensure optimal safe use of ARTISS it should be sprayed using a pressure regulator device that delivers a maximum pressure of up to 2.0 bar (28.5 psi). Prior to applying ARTISS the surface area of the wound needs to be dried by standard techniques (e.g. intermittent application of compresses, swabs, use of suction devices). Do not use pressurized air or gas for drying the site.
ARTISS must be sprayed only onto application sites that are visible. ARTISS should only be reconstituted and administered according to the instructions and with the devices recommended for this product.
When applying ARTISS using a spray device be sure to use a pressure and a distance from tissue within the ranges recommended by the manufacturer as follows:
Open wound surgery of subcutaneous tissue:
Spray set: Tisseel / Artiss Spray Set
Pressure regulator: EasySpray
Recommended distance from target tissue: 10 – 15 cm
Recommended spray pressure: 1.5-2.0 bar (21.5-28.5 psi)
When spraying the ARTISS, changes in blood pressure, pulse, oxygen saturation and end tidal CO2should be monitored because of the possibility of occurrence of air or gas embolism.
For the Posology, incompatibilities and interactions, please refer to your locally approved full SmPC.
Medicinal products are subject to medical subscription
Please see the Summary for Product Characteristics
Oct 2018